A5: Impact of microenvironmental factors on neutrophil effector functions directed against Salmonella (S.) enterica serovar Typhimurium
A5:Adaptive Responses of Neutrophils to Environmental Stressors in Acute Pathogen Defense
Neutrophils are the primary effectors in acute inflammation and pathogen defense. Equipped with a complex molecular arsenal, they must efficiently combat invaders even in challenging environments like abscesses or necrotic tissues. However, tightly controlling neutrophil function is crucial for tissue health: too much swarming can damage tissues, while insufficient swarming can promote infections. This project aims to explore how neutrophils adapt to such environments and integrate various signals to maintain effective defense responses.
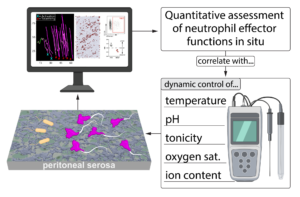
We have established an innovative experimental platform named “StromaScope” for studying tissue explants using multiple microscopy modalities. This platform enables us to visualize the interactions of neutrophils and other immune cells within tissues while controlling environmental parameters, such as temperature, pH, osmolarity etc.. By leveraging this technology, we investigate the process of neutrophil swarming, a highly dynamic response to pathogens or tissue damage. Our findings suggest that the initial decision to swarm is made by the first neutrophil to encounter a pathogen, termed the ‘pioneer neutrophil’. These pioneers undergo significant morphological changes, sometimes culminating in their death, which appears to trigger the swarming response.
We want to unravel the molecular mechanisms orchestrating the early decision-making of neutrophil swarming. Utilizing our tissue explant imaging system, we aim to delineate how environmental factors like pH changes and temperature fluctuations influence the onset of neutrophil swarming and their adaptive responses to acute environmental changes.
Our study will integrate both “wet” and “dry lab” approaches. We will employ various ex vivo cell culture systems with isolated murine or human neutrophils, including micro-fabricated “swarming chips” for preliminary screenings before proceeding to live tissue experiments and in vivo studies using our established imaging platform of the peritoneal serosa. In addition to generating high-quality images and videos, we will quantify cellular parameters such as neutrophil morphodynamics (dynamic cell shape changes over time) using advanced computational techniques, including machine learning and deep learning algorithms for data analysis. Ultimately, we aim to comprehend when, how, and why inflammation through neutrophil swarming begins in peripheral tissues and how tissue-specific factors modulate this process.
Supervisor
Prof. Dr. med. Stefan Uderhardt
91054 Erlangen

