B4: Acetate, a secreted metabolic product of Heligmosomoides polygyrus facilitates tissue invasion and maintains chronic infection
B4: Acetate, a secreted metabolic product of Heligmosomoides polygyrus facilitates tissue invasion and maintains chronic infection
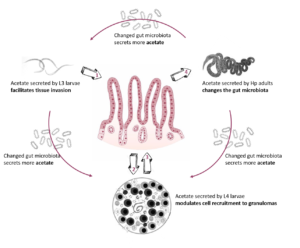
The immune system has co-evolved together with the almost ubiquitous intestinal parasitic helminths and large numbers of intestinal bacteria. During this co-evolution, intestinal helminths, such as Heligmosomoides polygyrus (Hp), have developed potent mechanisms to regulate their hosts’ immune response in order to continue their “symbiosis” and succeed chronic infections [1, 2]. We showed that local Hp infection in the intestine triggers additional systemic immunomodulatory mechanisms that attenuate autoimmune and allergic diseases [3, 4] in part by modulating the gut microbiota and its secreted metabolites [5]. During the first funding period, we identified that acetate, a metabolic product of Hp and the microbiota, facilitates host tissue invasion by Hp via intestinal epithelial barrier breakdown [6]. However, the role of acetate in the development of chronic helminth infections and underlying systemic immunomodulatory mechanism remains elusive. Our published data [6] together with recent preliminary results showing that Hp-derived acetate induce posttranslational protein modifications (PTM), specifically lysine acetylation in the micromilieu of the host tissue that surrounds the invading Hp larvae. Further, our preliminary data suggest that this Hp-induced local protein acetylation triggers specific anti-acetylated IgG and IgA systemic antibody secretion, which bind to the acetylated micromilieu as well as surface proteins of Hp larvae. In summary, we will build on our published results from the first funding period and investigate the consequences of Hp-induced protein acetylation.
References:
[1] Zaiss, M.M., et al., PLoS Pathog, 2013. [2] Mosconi, I., et al., Infect Immun, 2015. [3] Sarter, K., et al., Autoimmunity, 2017. [4] Zaiss, M.M., et al., Immunity, 2015. [5] Zaiss, M.M. and Harris, N., Parasite Immunol, 2016. [6] Schälter, F., et al., Int J Parasitol, 2022.
Supervisor
Prof. Dr. rer. nat. Mario Zaiss
91054 Erlangen
- Phone number: +49 9131 85-33794
- Email: mario.zaiss@fau.de
- Website: https://www.medizin3.uk-erlangen.de/forschung/arbeitsgruppen/ag-prof-dr-m-zaiss/

